On June 4, 2024, the FDA Psychopharmacologic Drugs Advisory Committee met to discuss the new drug application submitted by Lykos Therapeutics [for-profit public benefit corporation (PBC) initially part of MAPS (Multidisciplinary Association for Psychedelic Studies)] for MDMA in addition to psychotherapy to treat post traumatic stress disorder (PTSD). MDMA, otherwise known as midomafetamine, methylenedioxymethamphetamine, Ecstasy, or Molly is a Schedule I drug that shows great promise for treating otherwise very difficult to treat psychiatric conditions such as severe PTSD. The committee voted overwhelmingly against the effectiveness of MDMA for PTSD and voted that the benefits of this treatment do not outweigh the risks. In this episode, I unpack this decision, through the lens of all my potential biases as an emergency physician and toxicologist who also believes in the potential of psychedelic assisted psychotherapy. I'll be talking more about this topic in upcoming episodes, especially since the committee's decision is non-binding and the final FDA decision comes in August.
Please rate&review, share, and follow the podcast!
Instagram: @humanschoolpodcast & @patilarmenianmd
Music courtesy of Zach Effron, MD
From The Podcast
Human School
Working at the edge of life and death, in 2 medical specialties - emergency medicine and medical toxicology, Dr. Patil Armenian has seen a lot. Join Dr. Armenian as she shares stories & lessons (sometimes learned the hard way), interviews inspiring mentors, physicians, researchers & authors, and explores tools that can help navigate our busy lives. Though her philosophies were born in a busy Emergency Department, they apply to anyone trying to live a joyful life here on Earth. Welcome to Human School! Follow on IG @humanschoolpodcast & @patilarmenianmdJoin Podchaser to...
- Rate podcasts and episodes
- Follow podcasts and creators
- Create podcast and episode lists
- & much more
Episode Tags
Claim and edit this page to your liking.
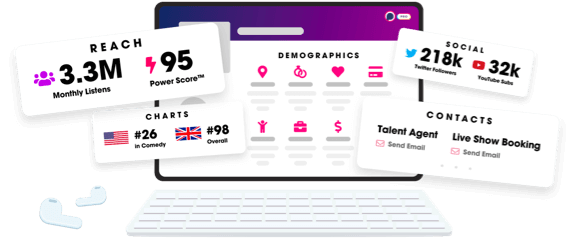
Unlock more with Podchaser Pro
- Audience Insights
- Contact Information
- Demographics
- Charts
- Sponsor History
- and More!
- Account
- Register
- Log In
- Find Friends
- Resources
- Help Center
- Blog
- API
Podchaser is the ultimate destination for podcast data, search, and discovery. Learn More
- © 2024 Podchaser, Inc.
- Privacy Policy
- Terms of Service
- Contact Us
